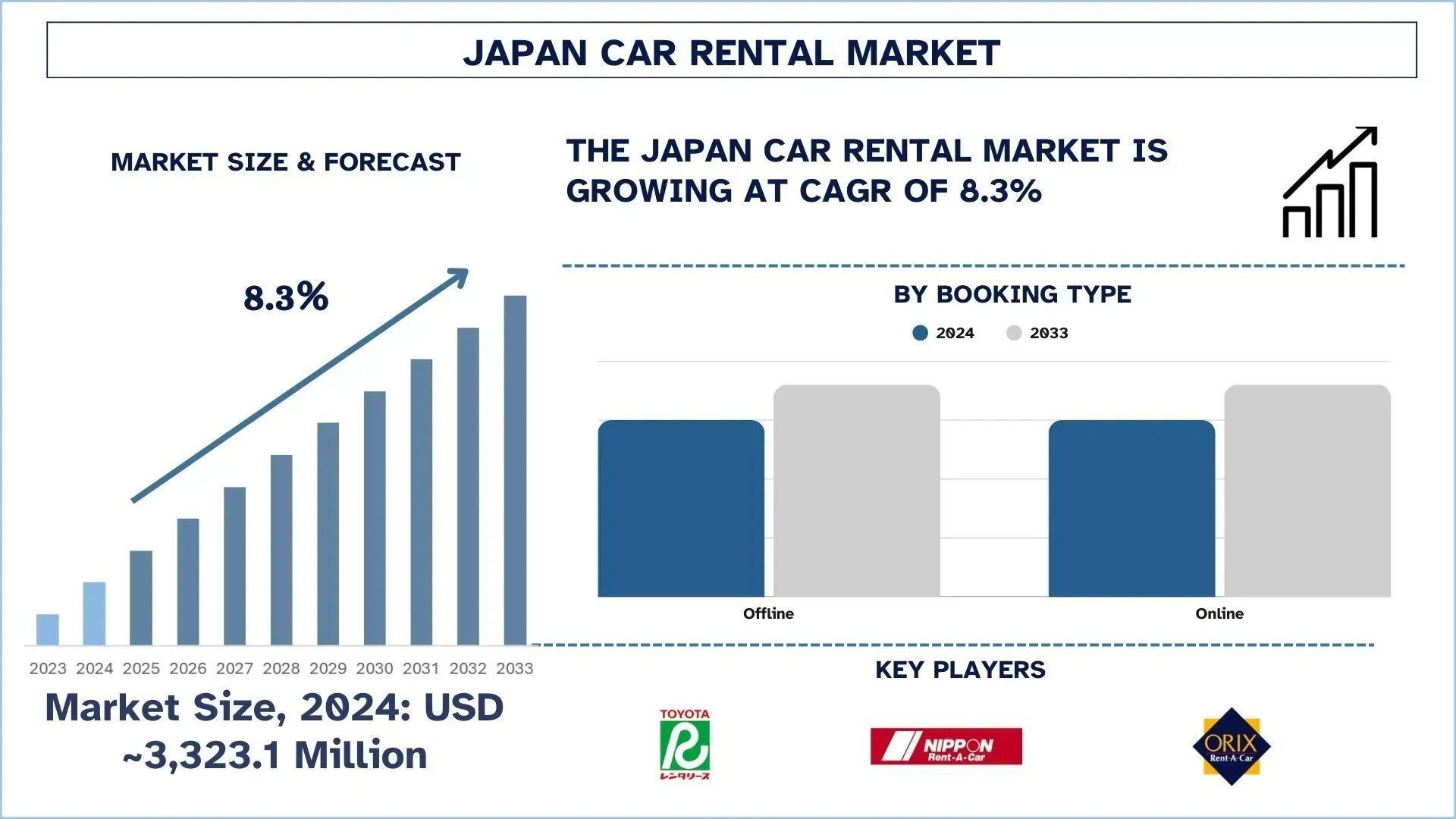
In Vitro Diagnostics Market Scenario Highlighting Global Trends, Challenges, And Emerging Opportunities
The in vitro diagnostics (IVD) market is an essential component of modern healthcare, providing tools for disease detection, monitoring, and treatment optimization. Understanding the current in vitro diagnostics market scenario is vital for healthcare providers, policymakers, and industry stakeholders. This scenario analysis explores the market’s trends, regional dynamics, challenges, and emerging opportunities shaping the global diagnostics landscape.
Global Market Overview
The IVD market encompasses a wide range of products and services, including reagents, instruments, software, and point-of-care testing solutions. These products are used across hospitals, laboratories, research centers, and home testing environments. The increasing prevalence of chronic and infectious diseases, coupled with advancements in diagnostic technologies, is fueling market expansion. Current market scenarios indicate steady growth, driven by both established and emerging economies.
Technological Innovations Shaping the Market
Technological advancements are at the forefront of the IVD market scenario. Molecular diagnostics, next-generation sequencing, and automated platforms are improving diagnostic accuracy and efficiency. Artificial intelligence and machine learning are increasingly integrated into data analysis, enabling faster results and predictive healthcare models. These innovations are transforming traditional testing methods, making diagnostics more reliable, scalable, and patient-focused.
Rise of Point-of-Care and Home Testing
The current market scenario shows a strong shift toward point-of-care (POC) and home testing solutions. POC testing provides rapid results at clinics, pharmacies, or bedside, allowing timely clinical decisions and better patient outcomes. Home diagnostics are gaining popularity due to convenience, affordability, and increasing consumer awareness of preventive healthcare. These trends highlight the growing emphasis on accessibility and patient-centered healthcare delivery.
Regional Market Scenario
The IVD market scenario varies across regions. North America continues to lead in adoption due to robust healthcare infrastructure, strong regulatory frameworks, and high technological integration. Europe follows, focusing on quality, innovation, and compliance. Emerging regions, including Asia-Pacific, Latin America, and parts of Africa, are experiencing rapid growth due to increasing healthcare investments, expanding infrastructure, and growing patient populations. However, affordability and accessibility challenges remain critical in these regions.
Challenges in the Current Market
The IVD market scenario is also defined by several challenges. Regulatory complexities and lengthy approval processes can delay product launches. High costs of advanced diagnostic technologies limit adoption, especially in low- and middle-income countries. Cybersecurity concerns related to digital diagnostics, infrastructure limitations, and inconsistent reimbursement policies further constrain growth. Addressing these challenges is essential for sustainable expansion and effective healthcare delivery.
Opportunities and Emerging Trends
Despite challenges, the market scenario presents numerous opportunities. Personalized medicine and biomarker-based testing are driving demand for specialized diagnostic solutions. Integration of digital platforms, AI, and telehealth enhances patient care and operational efficiency. Affordable and scalable testing solutions for emerging markets represent significant growth potential. Companies focusing on innovation, regional expansion, and patient accessibility can leverage these opportunities to strengthen their market presence.
Future Market Outlook
The in vitro diagnostics market scenario points to continued growth and transformation. Increasing emphasis on preventive care, rapid diagnostics, and personalized healthcare will shape future market strategies. Technological integration, global expansion, and collaborative partnerships will remain key to sustaining competitiveness. Companies that adapt to emerging trends, overcome challenges, and address patient needs are likely to achieve long-term success in this evolving market.
Conclusion
The in vitro diagnostics market scenario highlights a dynamic and evolving industry critical to global healthcare. Technological innovations, point-of-care and home testing, regional growth, and emerging opportunities define the current landscape. While challenges such as regulatory complexity, high costs, and cybersecurity risks exist, strategic planning, innovation, and accessibility initiatives can drive sustainable growth. By understanding the market scenario, stakeholders can make informed decisions, improve patient outcomes, and position themselves for success in a competitive and rapidly changing healthcare environment.