Ultomiris Drug Market: Revolutionizing Rare Disease Treatment with Next-Generation Therapy
The Ultomiris Drug Market is witnessing remarkable growth as healthcare advances bring new hope to patients suffering from rare and life-threatening diseases. Ultomiris (ravulizumab-cwvz), developed by Alexion Pharmaceuticals (a subsidiary of AstraZeneca), is a next-generation complement inhibitor that has redefined the management of diseases such as Paroxysmal Nocturnal Hemoglobinuria (PNH) and Atypical Hemolytic Uremic Syndrome (aHUS). With its longer dosing interval, superior efficacy, and expanding therapeutic applications, Ultomiris is setting new standards in the biopharmaceutical industry.
As the global focus on precision medicine and rare disease management intensifies, Ultomiris continues to gain traction among clinicians, payers, and patients. This blog delves into the market dynamics, key growth drivers, challenges, and future outlook of the Ultomiris Drug Market.
GET REPORT LINK:https://m2squareconsultancy.com/reports/ultomiris-drug-market
Ultomiris Drug Market Overview
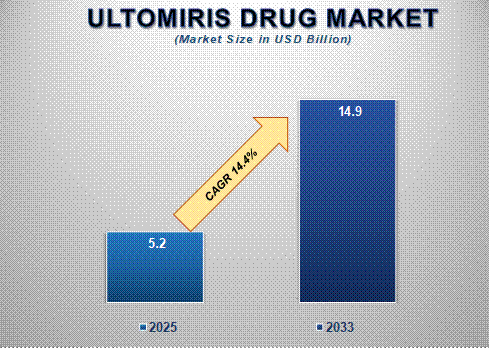
The global Ultomiris drug market size is poised for significant expansion, with its market size projected to grow from USD 5.2 billion in 2025 to USD 14.9 billion by 2033, reflecting a strong compound annual growth rate (CAGR) of 14.4% during the forecast period. The market’s rapid growth is driven by the increasing prevalence of these rare and life-threatening disorders, advancements in targeted therapies, and the drug's proven clinical efficacy in extending dosing intervals compared to previous treatments like Soliris.
Key Market Drivers
1. Growing Prevalence of Rare Diseases
Rare diseases like PNH and aHUS, though individually uncommon, collectively affect millions worldwide. The demand for effective, long-acting, and less burdensome treatments is growing as diagnosis rates improve due to genetic testing and awareness initiatives. Ultomiris offers significant quality-of-life improvements by requiring infusions every eight weeks, compared to every two weeks for its predecessor, Soliris.
2. Expanding Indications
One of the major growth accelerators for Ultomiris is its expanding therapeutic scope. Beyond PNH and aHUS, regulatory agencies in several regions have approved Ultomiris for conditions like Generalized Myasthenia Gravis (gMG) and Neuromyelitis Optica Spectrum Disorder (NMOSD). Clinical trials are also exploring its potential in additional complement-mediated disorders, which could unlock multi-billion-dollar opportunities.
3. Technological Advancements in Biologics
Continuous innovation in antibody engineering, formulation stability, and delivery mechanisms has supported Ultomiris’ evolution as a superior alternative to previous C5 inhibitors. These technological advancements have enabled longer half-life, improved patient adherence, and reduced healthcare resource utilization.
4. Strategic Collaborations and Investments
AstraZeneca and Alexion’s strategic collaborations with biotechnology firms, research institutions, and healthcare providers are fueling innovation and market penetration. Investments in R&D, real-world evidence studies, and patient-assistance programs are enhancing access and accelerating adoption worldwide.
5. Favorable Reimbursement and Regulatory Support
Many countries have introduced special frameworks for orphan drugs, offering incentives such as tax credits, market exclusivity, and expedited approvals. The favorable reimbursement environment for life-saving therapies like Ultomiris further supports strong market uptake.
TRENDING REPORTS:
https://m2squareconsultancy.com/reports/wearable-medical-devices-market
https://m2squareconsultancy.com/reports/global-energy-drinks-market
https://m2squareconsultancy.com/reports/std-diagnostics-market
https://m2squareconsultancy.com/reports/oncology-drugs-market
https://m2squareconsultancy.com/reports/ultomiris-drug-market
https://m2squareconsultancy.com/reports/sustainable-pharmaceutical-packaging-market
https://m2squareconsultancy.com/reports/syringe-infusion-pumps-market
https://m2squareconsultancy.com/reports/local-anesthesia-drugs-market
https://m2squareconsultancy.com/reports/hospital-acquired-infections-diagnostics-market
https://m2squareconsultancy.com/reports/biopolymers-and-bioplastics-market
https://m2squareconsultancy.com/reports/solar-panel-operation-and-maintenance-market
https://m2squareconsultancy.com/reports/fruit-and-vegetable-pulp-market
https://m2squareconsultancy.com/reports/spiritual-and-wellness-products-market
https://m2squareconsultancy.com/reports/portable-ultrasound-devices-market
https://m2squareconsultancy.com/reports/fecal-immunochemical-diagnostic-test-market
https://m2squareconsultancy.com/reports/next-generation-sequencing-market
https://m2squareconsultancy.com/reports/surgical-operating-tables-market
https://m2squareconsultancy.com/reports/floor-cleaner-market
Market Challenges
Despite its promising growth trajectory, the Ultomiris Drug Market faces several challenges:
-
High Cost of Therapy: Ultomiris remains one of the world’s most expensive drugs, making affordability and reimbursement key concerns for patients and healthcare systems.
-
Competition from Biosimilars and Emerging C5 Inhibitors: As patents for related biologics like Soliris expire, biosimilar development is intensifying, which could pressure Ultomiris’ market share.
-
Limited Patient Pool: Rare diseases by definition have small populations, restricting overall market size despite high per-patient costs.
-
Stringent Regulatory Processes: Gaining approval for new indications or formulations involves lengthy and costly clinical trials, which can delay commercialization.
REPORT SAMPLE LINK:https://m2squareconsultancy.com/request-sample/ultomiris-drug-market
BUY NOW:https://m2squareconsultancy.com/purchase/51
Regional Insights
-
North America: The region dominates the Ultomiris market, driven by strong healthcare infrastructure, high diagnosis rates, and favorable reimbursement policies in the U.S. and Canada. The U.S. FDA’s active orphan drug program further enhances the market potential.
-
Europe: Europe is a major market due to growing adoption in key countries like the UK, Germany, and France. The European Medicines Agency (EMA) has approved multiple indications, boosting regional growth.
-
Asia-Pacific: Rapid healthcare modernization, growing access to biologics, and increasing prevalence of rare diseases are propelling market expansion in Japan, China, and South Korea.
-
Latin America and Middle East: These emerging regions present untapped opportunities but face challenges related to drug pricing and limited rare disease infrastructure.
Future Outlook
The future of the Ultomiris Drug Market looks highly promising, with several growth avenues ahead:
-
Pipeline Expansion: Ongoing clinical trials for complement-mediated kidney diseases, autoimmune disorders, and neurological conditions could expand the product’s market base.
-
Subcutaneous and Self-Administered Formulations: Development of patient-friendly delivery options is expected to enhance convenience and compliance.
-
Digital Health Integration: Partnerships with digital platforms for remote patient monitoring and data-driven disease management will further strengthen market engagement.
-
Emerging Market Penetration: AstraZeneca’s strong global network is likely to drive Ultomiris adoption in developing economies through local partnerships and tiered pricing models.
Conclusion
The Ultomiris Drug Market stands at the forefront of innovation in rare disease therapeutics. By combining cutting-edge antibody technology with patient-centric design, Ultomiris has not only improved treatment outcomes but also transformed the care paradigm for patients with complement-mediated disorders. While high costs and competition pose challenges, continued R&D efforts, expanding indications, and global outreach strategies will ensure sustained market growth.
As the world moves toward more personalized and efficient healthcare solutions, Ultomiris serves as a benchmark for how science, innovation, and compassion can converge to redefine possibilities in modern medicine.
About m2squareconsultancy :
We are a purpose-driven market research and consulting company passionate about turning data into direction. Founded in 2023, we bring together researchers, strategists, and data scientists who believe that intelligence isn’t just about numbers, it’s about insight that sparks progress.
We cater to a wide range of industries by delivering customized solutions, strategic insights, and innovative support that help organizations grow, adapt, and lead in their respective sectors. Here’s a brief overview of key industries we work with.
Contact Us:
Email: sales@m2squareconsultancy.com
Phone (IN): +91 80978 74280
Phone (US): +1 929 447 0100
More Report:
https://m2squareconsultancy.com/reports/organic-personal-care-products-market
https://m2squareconsultancy.com/reports/portable-power-station-market
https://m2squareconsultancy.com/reports/power-transformer-market
https://m2squareconsultancy.com/reports/renewable-energy-market
https://m2squareconsultancy.com/reports/artificial-sweeteners-market
https://m2squareconsultancy.com/reports/blister-packaging-market
https://m2squareconsultancy.com/reports/digital-therapeutics-market
https://m2squareconsultancy.com/reports/drug-discovery-outsourcing-market
https://m2squareconsultancy.com/reports/e-pharmacy-market
https://m2squareconsultancy.com/reports/hospital-acquired-infections-diagnostics-market
https://m2squareconsultancy.com/reports/edible-oil-market
https://m2squareconsultancy.com/reports/solar-panel-operation-and-maintenance-market
https://m2squareconsultancy.com/reports/premium-chocolate-and-confectionery-market
https://m2squareconsultancy.com/reports/health-and-wellness-snacks-market
https://m2squareconsultancy.com/reports/fruit-and-vegetable-pulp-market
https://m2squareconsultancy.com/reports/dehydrated-vegetables-market
https://m2squareconsultancy.com/reports/spiritual-and-wellness-products-market
https://m2squareconsultancy.com/reports/portable-ultrasound-devices-market
https://m2squareconsultancy.com/reports/ophthalmic-lenses-market
https://m2squareconsultancy.com/reports/fecal-immunochemical-diagnostic-test-market
https://m2squareconsultancy.com/reports/sports-medicine-market
https://m2squareconsultancy.com/reports/military-drone-market
https://m2squareconsultancy.com/reports/almond-milk-market
https://m2squareconsultancy.com/reports/smart-shopping-cart-market
https://m2squareconsultancy.com/reports/drone-delivery-services-market
https://m2squareconsultancy.com/reports/smart-vehicle-market
https://m2squareconsultancy.com/reports/global-biopesticides-market
https://m2squareconsultancy.com/reports/financial-app-market
https://m2squareconsultancy.com/reports/cellular-iot-market
https://m2squareconsultancy.com/reports/supply-chain-security-market
https://m2squareconsultancy.com/reports/comic-book-market
https://m2squareconsultancy.com/reports/online-gambling-market
https://m2squareconsultancy.com/reports/beauty-tech-market
https://m2squareconsultancy.com/reports/global-exoskeleton-market







